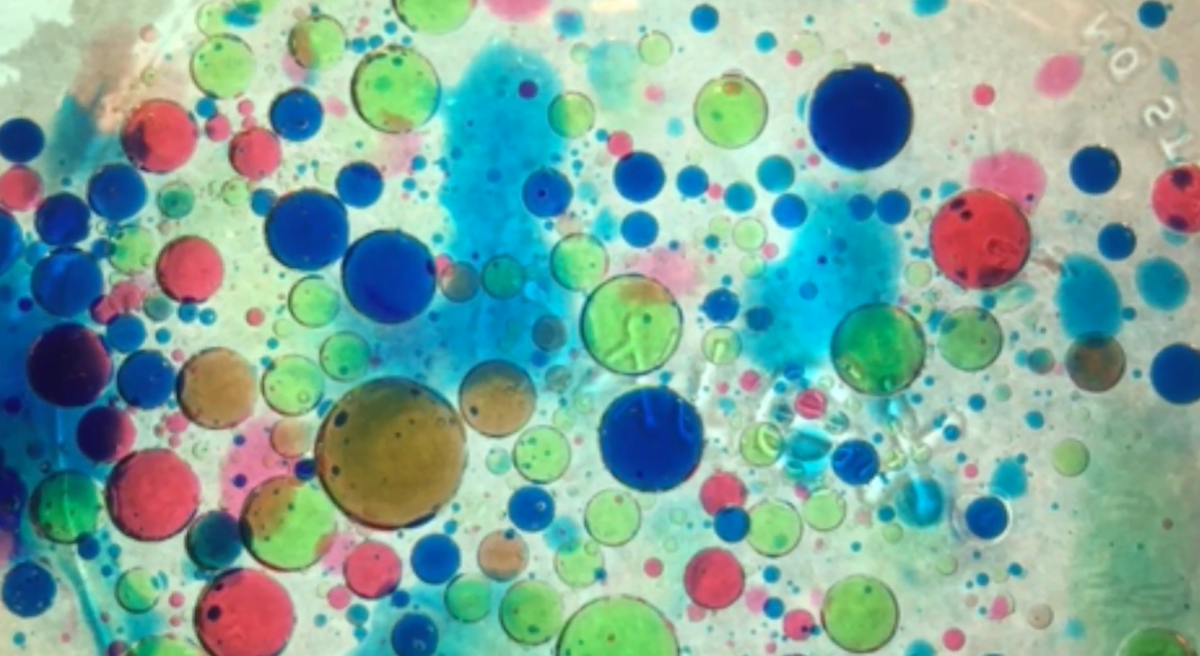
As part of our study of the properties of mixtures, we captured images of the chemical behavior of common household materials: vegetable oil, tap water and food coloring. As you view our photographs, notice that the bubbles are oil and the surrounding liquid is water, and that the polar water and the non-polar oil were immiscible; they did not mix to form a solution because their molecules are not chemically attracted to each other. The food coloring mixed with the water but not the oil, the molecules dispersed and dyed the water bright colors, they have high solubility and are hydrophilic substances. Look carefully and you will see there is a nonpolar layer of oil on the surface, as the oil is slightly less dense than the mixture below. There is a polar layer of dye and water, mixed together, creating a bright, vibrant kaleidoscope of colors and a fascinating effect described by Northwest Academy visual arts teacher Sean Cain as “oddly compelling.”
– Molly Sultany, Yasmin Godinez-Valencia and Nolan Hu